Defining Pharmaceutical Benefits Packages in Asia: A Comparative Analysis and Guide for Steps Forward
By Reva Alperson, Katie Shepard, and Andre Zida, USAID MTaPS
The core purpose of universal health coverage (UHC) is to ensure that all people can access the high-quality health care they need without suffering financial hardship. Establishing health benefits packages (HBPs) that include pharmaceuticals is a foundational step for governments to prevent financial hardship and protect individuals from catastrophic health expenditure.
HBPs are a set of health services and medical products that a particular group of beneficiaries is entitled to receive with specified financial protection as funded by the government or other coverage arrangement. Pharmaceutical benefits packages (PBPs) are an essential component of HBPs, pertaining to the set of covered pharmaceuticals for a group of beneficiaries. PBPs link medicines and commodities listed in the package to established sources of financing, thereby reducing out-of-pocket spending and the risk of catastrophic health expenditure.
Although there are many methods of deciding what is included in (or excluded from) PBPs, ideally, countries will systematically define pharmaceutical benefits based on the costs and benefits in relation to the country’s disease burden and financial resources. This intentional definition process helps maximize the cost-effectiveness of a country’s limited resources and achieve optimal levels of pharmaceutical access. HBPs and PBPs are instrumental in resource allocation processes that work toward meeting the population’s health needs and achieving UHC.
In recent decades, low- and middle-income countries in Asia have developed substantial health financing and coverage arrangements; however, many countries do not offer financial risk protection, face financial barriers to accessing health care services, and experience high rates of catastrophic health expenditures. For PBPs in particular, there is need for stronger value-based resource allocation and a more detailed understanding of how Asian countries define pharmaceutical benefits (if at all).
Two New Resources Offer Guidance for Health Policymakers
As part of the USAID Medicines, Technologies, and Pharmaceutical Services (MTaPS) Program, Results for Development (R4D) recently published two reports:
- “Pharmaceutical Benefits and Benefits Packages in Asia: A Cross-Country Mapping of Coverage Arrangements” – This report analyzes key pharmaceutical coverage and financing arrangements in 14 Asian countries to categorize and compare countries’ definitions of HBPs and pharmaceutical benefits, including how medicines are incorporated in provider payment mechanisms and the degree of cost-sharing.
- “Brief: Key Steps for Defining Pharmaceutical Benefits Packages” – This brief functions as a guide for policymakers to establish PBPs within their health benefits policy.
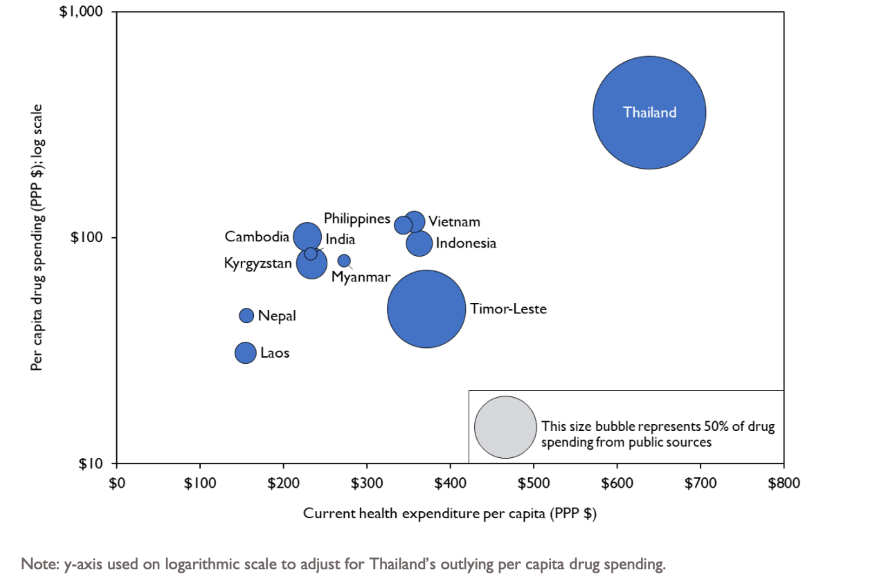
Our analysis found that 11 of the 14 selected countries in Asia (79%) defined pharmaceutical benefits coverage through a variety of approaches. Within these countries, there is limited use of explicitly defined benefits packages that name and quantify the drugs included in the package, create legal entitlements to that package, and outline financing arrangements for the included pharmaceuticals. In fact, private expenditure constituted an average of 72% of financing sources for pharmaceuticals across the 14 countries analyzed, the majority of which were out-of-pocket payments. As shown in figure 1, higher per capita current health expenditure was associated with higher per capita expenditure on pharmaceuticals on average, but there was not a clear pattern in how much of pharmaceutical expenditure was from public sources.
Figure 1: Per capita pharmaceutical expenditure versus per capita current health expenditure (CHE) and relative proportion of pharmaceutical expenditures from public sources (represented by size of bubbles)
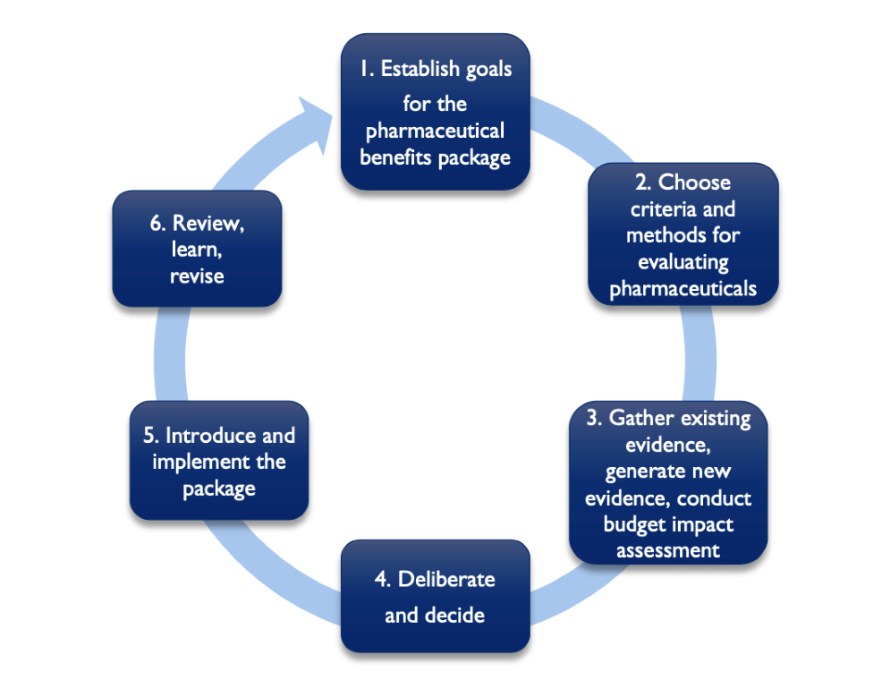
In light of the clear benefits of PBPs (and the need for more explicitly defined PBPs in Asia), the second report describes the following steps (building on work done by Glassman et al. ) for countries to define PBPs while highlighting best practices from Thailand, Indonesia, and the Philippines.
Figure 2: Defining a Pharmaceutical Benefits Package
Some Key Findings and Conclusions
- While Asian countries overall lack explicitly defined PBPs, there are promising examples to learn from and clear opportunities to improve pharmaceutical benefits coverage.
- The PBP definition process is an important policy tool for ensuring efficient use of limited public resources, financial protection, and UHC.
- It is most effective when it incorporates stakeholders from a variety of backgrounds, both inside and outside the government, from clinicians and pharmacists to civil society groups and policymakers.
The resources put forth by the MTaPS Program offer a path forward based on the current landscape of PBP definition processes in Asia. Although refining a country’s PBP process will take time, the framework for defining PBPs, and lessons from the published country examples can serve as a preliminary step in a positive direction.