The Challenge
COVID-19 has been a defining global health crisis in our journey to stronger health systems. When the outbreak of the novel coronavirus started in early 2020, it became an urgent call to rapidly mobilize responses in countries to mitigate its effects. But since then, responding to the pandemic has also opened opportunities on the sidelines to further strengthen health systems in countries for long-lasting gains like greater resilience and equitable, quality health care delivery—the goal of USAID.
Committed to fighting the pandemic and building back better, USAID tasked its Medicines, Technologies, and Pharmaceutical Services (MTaPS) program to be a partner in USAID’s global response to the pandemic.
MTaPS’ Technical Areas of Support
To support USAID’s global pandemic response, MTaPS provided rapid technical assistance to 13 countries. MTaPS applied evidence-based approaches and drew upon the multisectoral coordination networks and partnerships developed through its infection prevention and control (IPC) work under the Global Health Security Agenda (GHSA) to rapidly build capacities of the health workforce for mitigating and managing infections. Upon approval of COVID-19 vaccines for immunization, MTaPS applied its pharmaceutical systems expertise across the spectrum—from regulatory and policy support to introduce new health technologies (such as vaccines and diagnostics) to procurement and supply chain logistics management, vaccine safety monitoring, and human resources capacity development to facilitate COVID-19 vaccine deployment.
MTaPS is aligned with the U.S. COVID-19 Global Response and Recovery Framework and supports two objectives:
Objective 1: Accelerate equitable
access to and delivery of safe
and effective COVID-19
vaccinations
Objective 2: Reduce morbidity and mortality from COVID-19, mitigate transmission, and strengthen health systems, including future pandemic response

- Policy, regulation, coordination, and implementation planning
- Guides and tools for vaccine introduction
- Vaccine-related human resources capacity building
- Vaccine safety monitoring and reporting
- Safe vaccine services delivery, IPC, and waste management
- Vaccine procurement, supply chain, and logistics
- Risk communications and advocacy
- Coordination and operations for COVID-19 response and mitigation
- COVID-19 infection prevention and control capacity building from national to health facility level
- Monitoring COVID-19 IPC standards compliance at facilities
- Integrating continued IPC improvements in health systems
- Managing COVID-19 IPC commodities at the national and facility level
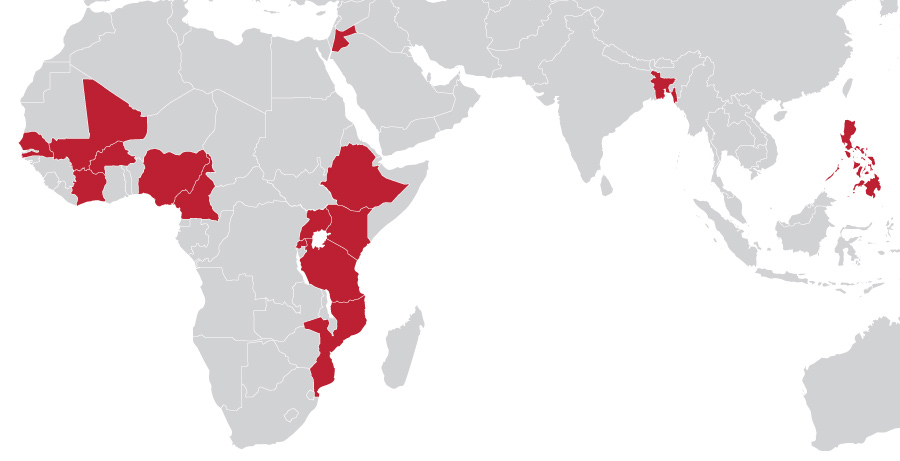
Countries of COVID Support
Impact in Numbers
Developed COVID-19 Infection Prevention and Control (IPC) Capacities in 13 Countries (data as of September 2023)

49,278
Number of workers trained on
COVID-19-related IPC and/or WASH
6,254
Number of health facilities supported for IPC and/or WASH for COVID-19

29
Number of COVID-19 e-learning modules developed in English and French (some accessible on LeaderNet.org)
Supporting Introduction and Deployment of COVID-19 Vaccines in 11 Countries (data as of June 2023)
34
Number of COVID-19 vaccine multisectoral coordination mechanisms that meet regularly

153
Number of policies, protocols, standards, and guidelines developed or adapted for COVID-19 vaccine deployment

5
Countries that improved the regulatory and/or policy environment for COVID-19 vaccines with MTaPS' support

4
Countries that developed or adapted COVID-19 vaccine micro plans with MTaPS' support

9,406
Number of adverse event following immunization (AEFI) reports reviewed with MTaPS support

12,817
Number of people trained on COVID-19 vaccine-related topics




