Regulating COVID-19 Medical Products: An Urgent Need
COVID-19 has triggered an urgent need for new medical products, including vaccines, medicines, diagnostics, personal protective equipment (PPE), and other medical devices such as those that deliver oxygen. As researchers rush to develop these products and clinicians plan for their delivery and safe use, national regulatory authorities play an essential public health role in ensuring that these products are safe, effective, quality-assured and affordable, and accessible to those in need in a timely manner. The USAID MTaPS Program is harnessing its regulatory systems strengthening exper tise to suppor t low- and middle-income countries in ensuring that COVID-19 medical products meet regulatory standards and save lives as intended.
What is needed?
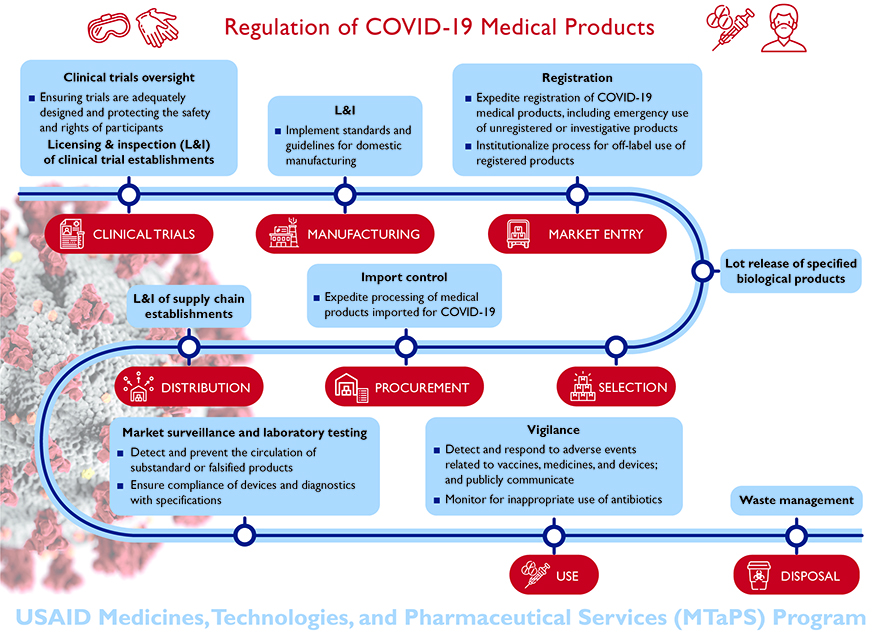
The infographic below highlights vital regulatory functions needed to ensure safe, effective, and quality-assured COVID-19 medical products in LMICs and beyond.
The USAID Medicines, Technologies, and Pharmaceutical Services (MTaPS) Program enables low- and middle-income countries to strengthen their pharmaceutical systems, which is pivotal to higher-performing health systems. MTaPS focuses on improving access to essential medical products and related services, and the appropriate use of medicines to ensure better health outcomes for all populations. The program is implemented by a consortium of global and local partners and led by Management Sciences for Health (MSH), a global health nonprofit.
For more information, contact: Kate Kikule, MTaPS Principal Technical Advisor-Pharmaceutical Regulatory Systems, at [email protected]