Managing Conflicts of Interest: A Practical Application in Pharmaceutical Sector Committees
Conflict of interest (COI) is a significant issue in the governance of pharmaceutical systems, affecting not only the quality of medicines that are delivered but also the cost to the health system. On October 4, 2022, global experts convened to discuss this prevalent issue and the ongoing research behind it, and to help countries to manage the issue practically by looking at the newly released how-to guide developed by the World Health Organization (WHO) in collaboration with the USAID Medicines, Technologies, and Pharmaceutical Services Program (MTaPS).
“We’re needing to fight an increasing burden of chronic diseases which affect or kill 40 million per year,” said Taryn Vian, Professor at the University of San Francisco, California, as she highlighted the need to strengthen governance and meet the WHO’s Sustainable Development Goals. With good governance, “we can ensure that health resources are being used wisely, fairly, not wasted or lost to corruption; and in doing this, we promote and maintain trust in health care systems.”
A study published in the BMJ in 2021 examined more than 500 published studies in 57 countries, where it found extensive private-sector ties to activities and parties in the health care ecosystem, underlining the following realizations:
- The private sector plays an important role in the provision of essential medicines.
- Pharmaceutical committees often rely on industry for technical knowledge and expertise.
- Private sector actors may influence decision-making processes at multiple points.
A conflict of interest is defined as a situation and a risk that arises when a person has a financial or other interest that could potentially interfere with their entrusted obligation, duty, or responsibility to serve a party or perform a role. COI, particularly those arising from financial or contractual relationships between private-sector entities and public pharmaceutical-sector committee members, have been shown to compromise the integrity of decision-making, impacting public health and public health budgets.
“Managing conflicts of interest: A how-to guide for public pharmaceutical-sector committees in low- and middle-income countries,” or the COI manual, focuses on public pharmaceutical committees and agencies to help close gaps in the integrity of their decision-making processes.
“The impetus for developing this manual really came from countries who were requesting support,” said Deidre Dimancesco, Technical Officer with the WHO Department of Health Products Policy and Standards and one of the lead contributors to the manual. “[The manual] does contain proposed actions that can be adapted to different country contexts, and while it focuses on pharmaceuticals, it may also be relevant for other health products,” continued Dimancesco.
The Managing Conflicts of Interest How-To Guide
The COI manual highlights 10 critical steps for improving COI policy, prevention, and management in public pharmaceutical decision-making committees. These steps are built upon four overarching principles of managing COIs: proportionality, transparency, accountability, and fairness.
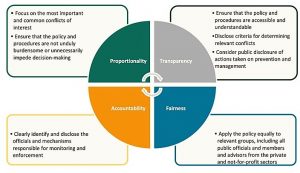
General principles to guide the development, implementation, and evaluation of conflict of interest policies for public pharmaceutical committees (from Managing conflicts of interest, 2022, page 11)
The practical steps include strategies for policy and implementation as well as for prevention and management processes. The steps mark a starting point for evaluating what currently exists in a country and how to build on or develop COI policies and processes. From there, the manual offers steps on approaches for training, monitoring, and enforcement mechanisms and activities to raise awareness and encourage policy compliance. Lastly, the steps outline processes and procedures at the committee level to identify and prevent COI and respond when situations arise.
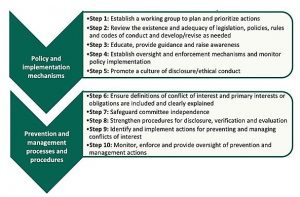
Ten steps for improving the prevention and management of conflicts of interest. (from Managing conflicts of interest, 2022, page 10.
The session also highlighted the findings of another qualitative study on COI management and policies and practices in public pharmaceutical sectors in Southeast Asia. The report pulled data from 41 committees in 10 countries: Bangladesh, Bhutan, India, Indonesia, Maldives, Myanmar, Nepal, Sri Lanka, Thailand, and Timor-Leste. The study found that although there is strong awareness of COI and the committees have consistent disclosing requirements, there is little guidance on how to manage the disclosed information and little emphasis on prevention of COI, making the new manual even more relevant.
Examples from Countries
What do COI challenges look like in practice? As a member of the National Pharmaceutical Regulatory Agency in Malaysia, Nicholas Leow explains the challenges faced by the regulatory body: “Being the gatekeeper to products that are going to be registered in Malaysia, we do face quite a lot of pressures from parties with vested interests to get the products approved or at least get an expedited evaluation of the product.” Leow also noticed that on committees within hospitals and universities, pharmaceutical experts are more likely to approve grants and research that can either affect research funding or raise the status of the organization. To combat this, the agency now holds regular meetings to increase awareness around COI, involving both technical and organizational stakeholders, to ensure that there is a multi-tier effort in the decision-making process.
In Uganda, the challenges stem from a competing labor market low in registered pharmaceutical professionals and saturated with pharmacies. Brian Sekayombya of Uganda’s National Drug Authority highlighted the techniques and processes to build a culture that is constantly working to prevent COI in the hiring process.
“Be on the constant lookout where the potential areas [of conflict of interest are],” said Sekayombya, while emphasizing the need to recognize natural human behaviors embedded within the culture.
What’s Next
The groundwork for managing COI in pharmaceutical committees marks the first step for countries to move toward stronger pharmaceutical systems. e-Learning materials are currently being developed by MTaPS to support the manual, with additional research to be conducted and continuous elaboration of the manual as more evidence is gathered.