Pharmaceutical Pricing Policies in Asia: Promoting Affordability
By Reva Alperson and Ioana Ursu, Results for Development, USAID MTaPS
As the rise in health expenditures outpaces economic growth worldwide, consumers in Asian and Pacific countries without universal health coverage continue to face high out-of-pocket (OOP) costs, which account for approximately half of health care spending in these countries. Because pharmaceuticals make up 25% of total health expenditure and, typically, an even larger share of OOP costs (OECD Health at a Glance 2019), it is essential to control pharmaceutical costs as a key step toward universal health coverage.
Many Asian countries have developed pharmaceutical pricing policies to contain the rising costs of medicines financed by the public sector. The World Health Organization (WHO) defines a pricing policy as “a set of written principles or requirements for managing the prices of pharmaceutical products, agreed or adopted by a public institution (e.g., a government authority), a group of purchasing organizations, or individual health services.” Pricing policies usually target supply-side issues by influencing the price of pharmaceuticals from manufacturers, wholesalers, and retailers, for example.
Pharmaceutical pricing policies come in many forms with ranging complexity and varying amounts of information available in the public domain. To centralize this information, the USAID Medicines, Technologies, and Pharmaceutical Systems (MTaPS) program published a report comparing pharmaceutical pricing policies across 11 Asian countries by using secondary desk research and qualitative primary research. These countries—Bangladesh, Cambodia, India, Indonesia, Kyrgyz Republic, Myanmar, Nepal, The Philippines, Sri Lanka, Timor-Leste, and Vietnam—currently receive health funding from USAID’s Asia Bureau.
The “Review of Pricing Policies and Price Lists Available in Asia Regional Countries” report aims to:
- Provide a catalog of the pricing policies currently used in the targeted countries
- Document the types of publicly available information on manufacturer, wholesale, and retail prices in these countries
- Review the extent to which indexes and international reference pricing rules are being designed and used to standardize purchase prices and negotiate the best value for such products
- Assess whether country-level pricing policies are normative in nature and are consistently followed and implemented by purchasing organizations
Findings
Listing of Medicine Price Lists
Of the 11 countries reviewed, 8 publish some form of medicine price list, and of these, only India and Vietnam publish prices for all medicines registered in the country. All other countries with price lists include the prices for only medicines with a price control. Some share the price lists only with public facilities. Because most of the published prices are set at the retail level rather than by manufacturers, this poses challenges to comparing prices across countries, as retail prices reflect country-specific taxes, distribution, and retail margins. These costs reflect economic and geographical disparities between countries, and therefore limit the ability of neighboring countries to leverage this information to negotiate manufacturers’ prices.
Pharmaceutical Pricing Policies in Use and Transparency
The report documents 11 supply-side pricing policies stemming from the 2020 WHO guidelines on pricing policies and the 2019 WHO report on pricing of cancer medicines, ordering them on their relative complexity in the policies’ regulation and implementation (figure below). Countries are also placed along the continuum by the number and complexity of policies they use, based on profiling and assessment of each country’s approach to pharmaceutical pricing policy.
Classification of pharmaceutical pricing policies according to complexity of implementation (increasing from left to right)
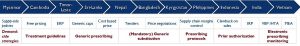
ERP, external reference pricing; IRP, internal reference pricing or usage of therapeutic class comparison; VBP, value-based pricing (usage of HTA approaches); MEA, managed entry agreement
The analysis showed that the most common pricing policy used by all 11 countries was tendering in the public sector, centralized at the national or regional level, and usually coupled with local tendering by public facilities. However, only two countries (Indonesia and the Philippines) make the results of tenders publicly available, and thereby ensure they are accessible to private facilities. The lack of information on tendering raises questions about the accountability, transparency, and fairness of the process in those countries and limits the market’s ability to pressure prices downward across both the public and private sectors. Since the winning prices of the tendering process are rarely made available to the whole market, tendering offers limited help in pharmaceutical price control. Similar issues with lack of transparency affect external and internal reference pricing systems (which were used by half of the countries reviewed) and hinder implementation of these pricing policies by purchasing organizations.
Report Shares Some Practical Takeaways
The report details further insights on specific pricing policies that broadly suggest practical approaches for effective pharmaceutical pricing. Key findings include the following:
- Although multiple pricing policy interventions should be used, there is no one combination of policies that can be uniformly applied to ensure wide access, affordability, and sustainability of health systems, and any such mix should take into consideration the population’s needs, local market dynamics, and available finances. However, there is a correlation between wider access to more medicines and the use of more, rather than fewer, pricing interventions.
- Following the recommendation of WHO, countries should apply policies that control the margins or maximum mark-ups that can be added to the manufacturer’s price throughout the supply chain.
- Building a regional database of prices can guide governments in benchmarking their prices to pursue internal or external reference pricing policies.
The report serves as a practical basis for policymakers in Asia to learn about the various approaches to pharmaceutical pricing policy and compare what strategies have and have not been successful in their region. With this information, countries can share experiences and best practices with their neighbors with similar approaches to pricing policy, paving the road for more affordable access to pharmaceuticals and universal health coverage.