Streamlining Product Registration to Ensure Increased Access to Safe, Quality, and Effective Medicines in Nepal
In Nepal, the Department of Drug Administration (DDA) is responsible for ensuring access to safe, quality, and efficacious medicines by regulating drug production, registration, and distribution. In the past years the DDA had built up an extensive backlog of product registration applications due to insufficient personnel and the high complexity of the registration process (figure 1). Such bottlenecks prevented medicines from reaching the Nepal market, including new, innovative products, and contributed to increased risk of supply chain disruptions.
With MTaPS support, DDA expedited the registration process of 100 products which were pending for years contributing to improving the availability of medical products in the country.
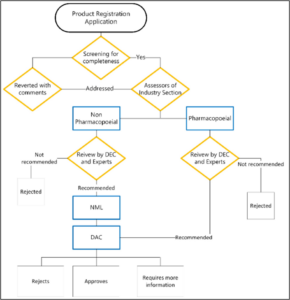
Figure 1: Product registration process in Nepal
DEC: drug evaluation committee, DAC: drug advisory committee, NML: national medicine laboratory
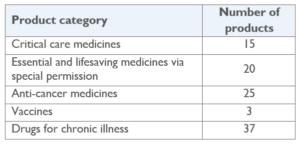
Table 1: Products expedited for registration (2021-2022)
Strengthening Medical Product Registration and Market Authorization
MTaPS assisted the DDA to expedite and simplify the product registration and market authorization processes in several ways. For instance, based on common technical document for dossier evaluation, DDA reviewed the registration checklists and integrated it into its DDA-Medicines Information System (MIS) product registration module. Moreover, DDA dedicated a staff to revise the registration checklist. DDA’s digitalization shall reduce the amount of paperwork and the risk of errors. Once fully implemented, the DDA-MIS will allow the DDA to regulate the manufacturer more robustly and process product registration applications and product authorizations more efficiently. For example, the time taken to evaluate a product is estimated to decrease from an average of five weeks to two weeks for products that are categorized as lifesaving, essential, and critical care; whereas, for other products, it is expected to go from several months to weeks.
From July 2021 to April 2022, MTaPS supported the DDA to successfully evaluate 80 products for registration and 20 for special import permission (i.e., unregistered but vital products such as orphan drugs) which contributed to address the registration buildup and cleared the way for providing healthcare professionals with more treatment options and increased availability of life-saving products to the people of Nepal.
Additionally, DDA identified 18 out of the 100 products evaluated which did not meet the required quality, safety, and efficacy standards and were ineligible for registration contributing to protect public health and ensure safety of the population in Nepal.
“The evaluation of these products for registration and special permission has played a crucial role in improving the availability, accessibility, and quality of essential, lifesaving, critical care, and lifestyle medications throughout the country, positively impacting public health and patient care.”—Mr. Umanga Tripathee, Pharmacy Officer and Secretary of the Drug Evaluation Committee
Continuing Impact
In response to the disrupted health supply chain that Nepal and the rest of the world experienced during the Covid-19 pandemic, the Ministry of Health and Population and DDA have encouraged local drug manufacturers to boost their production to self-sustain the demand for essential and lifesaving drugs. The success of this initiative, however, relies on an expeditious product evaluation and registration process reducing the burden of backlog and shortened the average time taken for registration and authorization of medical products.
