Strengthening Clinical Trial with Inspection Readiness
The Department of Drug Administration (DDA) is a regulatory authority responsible for oversight of clinical trials in Nepal as per the Drug Act 1978. The Nepal Health Research Council (NHRC) is a national body that oversees high-level study and research on health as per the NHRC Act 1991. Although these Acts are in place, the legal provisions to regulate clinical trials are incomplete and the authorities lack the necessary tools to authorize, suspend or stop clinical trials, such as guidelines, standard operating procedures (SOPs), inspection tools, and adequately trained personnel. The current clinical trial approval process in Nepal is presented in figure 1.
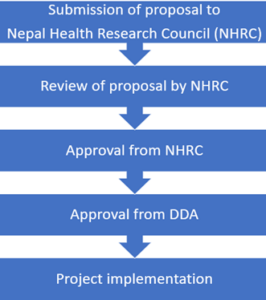
Figure 1: Clinical Trial Approval Process in Nepal
MTaPS supported the DDA in strengthening the regulation of clinical trials with the aim of protecting participants’ rights, ensuring scientific integrity, and facilitating the development of new medical treatments. A World Health Organization (WHO) Global Benchmarking Tool (GBT) assessment of the DDA in March 2021 recommended the revision of the legal framework and the formulation of SOPs and guidelines to increase the regulatory maturity for clinical trials. To address this, DDA implemented a multipronged approach to strengthen clinical trial regulation (figure 2) while maintaining a balance between participants’ rights and promoting medical progress while ensuring the highest level of safety and ethical conduct in clinical research.
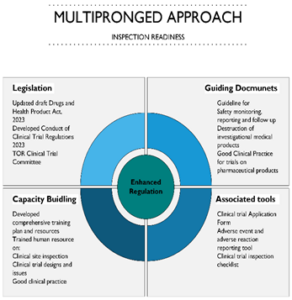
Figure 2: Multipronged approach for implementation of clinical trial inspection by DDA in Nepal
Improving Regulation of Clinical Trial
Legislation and guiding documents
MTaPS supported DDA, between March 2022 and June 2023, to develop the clinical trial regulation, terms of reference for the clinical trial committee, guidelines, and SOPs for regulating clinical trials in Nepal. As part of this, a clinical trial application form, reporting tool for adverse events and adverse reactions, and guidelines for clinical trial inspection were also developed. In May 2023 the DDA approved the clinical trials inspection guideline which includes the checklist. Other documents including the regulations are yet to be approved.
Capacity building of DDA
MTaPS supported DDA to train their workforce in clinical trial design, good clinical practice, and inspection of trial sites. DDA trained four staff to detect, collect, assess, report, and respond to potential concerns associated with clinical trials such as safety issues linked to the investigational medical products.
Clinical trial site inspection by the DDA
DDA conducted its first clinical trial inspection at a site in Kathmandu in June 2023 using the checklist and trained personnel supported by MTaPS. Based on the approved checklist, DDA conducted additional audits at two sites to determine the robustness of the checklist. After the inspection, the checklist proved to be comprehensive enough to cover all major activities such as legal and administrative approvals, informed consent forms, investigational medical products, ethical approval, data recording, study report and their respective documentation.
“DDA is very proud to state that it conducted the first-ever regulatory audit of the clinical trial site on June 13, 2023. The recently approved clinical trial site inspection guideline has been instrumental to that effect.” Mr. Umanga Tripathee, Pharmacy Officer and Contact Person Clinical Trials
Continuing Impact
DDA continued conducting clinical trial inspections usinng the tools developed in collaboration with MTaPS. These inspections are vital for safeguarding the interests of participants, maintaining data integrity, and contributing to the advancement of medical science in Nepal and worldwide.