Tools and Practices to Leverage Private Sector Logistics Services to Enhance Performance of Public Sector Health Supply Chains
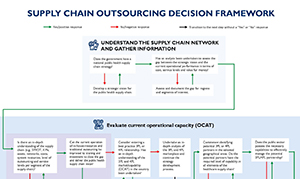
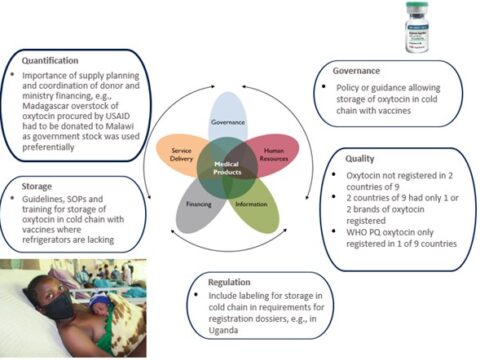
Publish : 08th Feb 2024Background: Despite significant improvements in public health supply chains in recent decades, low- and middle-income countries (LMICs), such as Nigeria and the Philippines, still face numerous […]
Read MoreHow Pharmaceutical Systems Strengthening Benefits Maternal, Newborn, and Child Health Care Services
Publish : 30th Jan 2024By Jane Briggs, USAID MTaPS, and Patricia Jodrey, USAID Global trends in maternal, newborn, and child health (MNCH) had been increasingly positive; for example, worldwide, maternal […]
Read MoreEstimating needs of non-malaria commodities for Integrated Community Case Management (iCCM)
Publish : 22nd Jan 2024Une traduction de cette page est disponible en français. Pour effectuer cette traduction, veuillez cliquer en haut à droite du site web sur « Select Language » puis […]
Read More