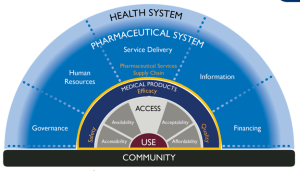
Approaches and Tools for Strengthening Pharmaceutical Systems
Publish : 28th Feb 2023Strong pharmaceutical systems are critical for ensuring access to and appropriate use of safe, effective, quality-assured, affordable medical products and related services to improve health. Pharmaceutical […]
Read MoreOvercoming the Barriers to Entry: How to Improve the Registration Process for Essential Medicines in Low- and Middle-Income Countries?
Publish : 19th Jan 2022Ineffective and inefficient registration processes impede access to lifesaving, quality-assured essential medicines. Inadequate legal provisions, limited capacity within National Regulatory Authorities, and long and unpredictable registration […]
Read MoreRegional Harmonization of Medicines Regulation: Reaping Benefits Beyond Borders
Publish : 01st Dec 2021USAID MTaPS hosted an event titled “Regional Harmonization of Medicines Regulation: Reaping Benefits Beyond Borders” on November 10, 2021. The objective of this event was to […]
Read More