Approaches and Tools for Strengthening Pharmaceutical Systems
Strong pharmaceutical systems are critical for ensuring access to and appropriate use of safe, effective, quality-assured, affordable medical products and related services to improve health. Pharmaceutical systems strengthening (PSS) is the process of identifying and implementing strategies and actions that achieve coordinated and sustainable improvements in the critical components of a pharmaceutical system to make it more responsive and resilient and to enhance its performance for achieving better health outcomes.
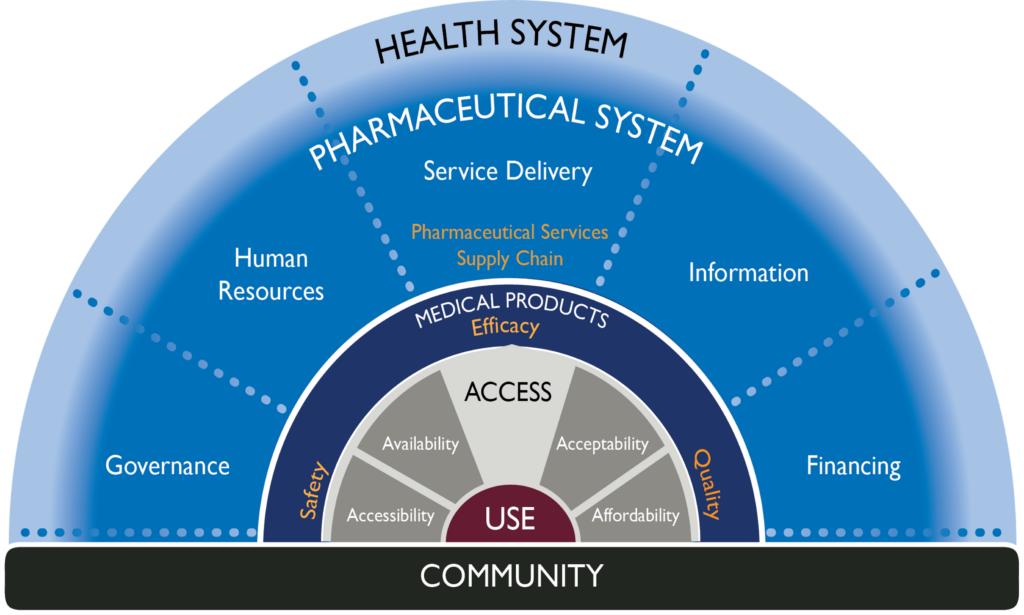
Figure 1. A pharmaceutical system consists of all structures, people, resources, processes, and their interactions within the broader health system that aim to ensure equitable and timely access to safe, effective, quality pharmaceutical products and related services that promote their appropriate and cost-effective use to improve health outcomes.
As part of its support to low- and middle-income countries, MTaPS uses various approaches and tools to help countries achieve better health outcomes and higher-performing health systems by improving the overall effectiveness of pharmaceutical systems through improving individual systems components.
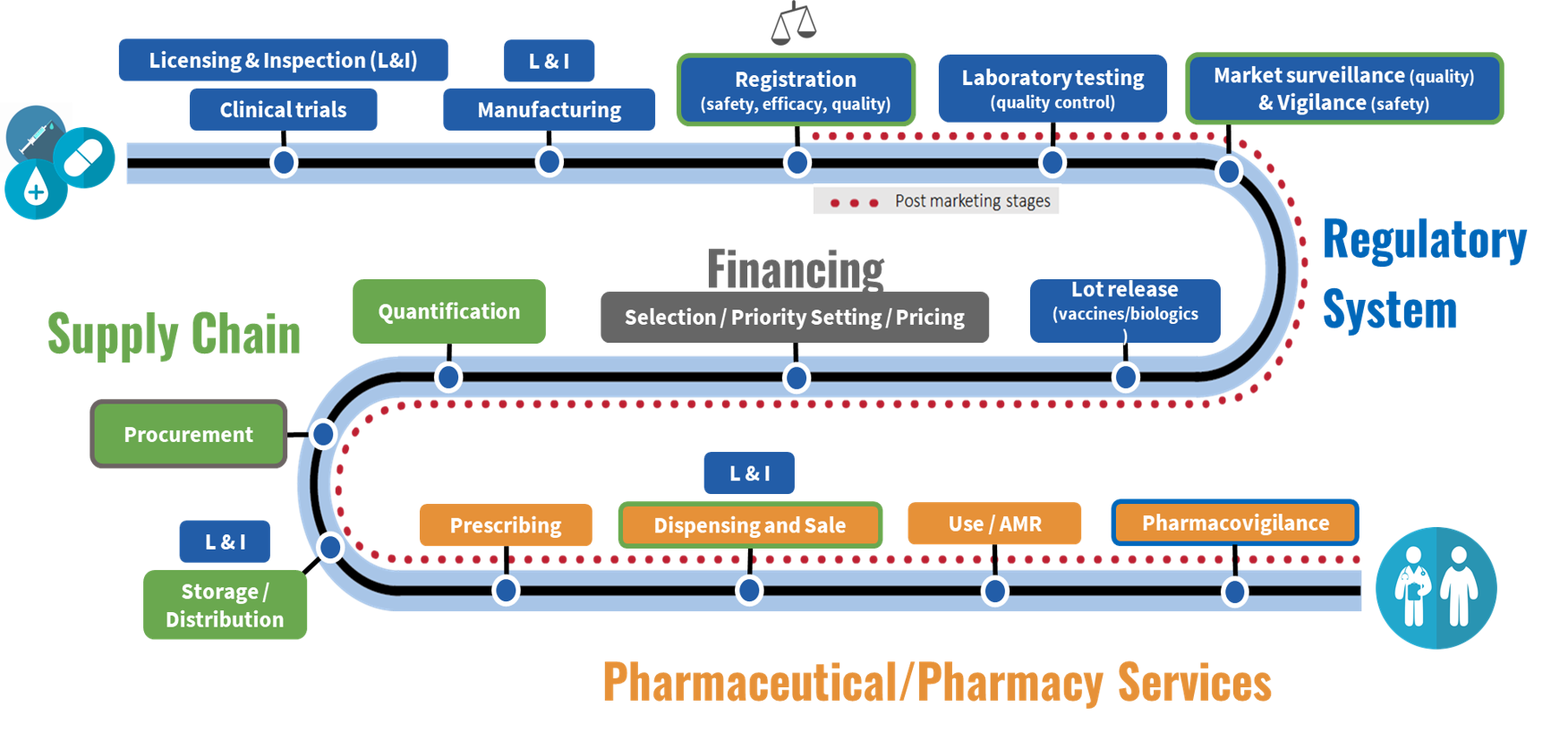
Local organizations—including Ministries of Health, academic institutions, professional associations, and local implementing partners—are taking an increasing lead in the improvement of pharmaceutical systems in their country context. Below, we present a library of resources across nine different areas of PSS that can be applied in specific country contexts.
For more information about the East Africa PSS Skills Exchange held February 28-March 2, 2023, click here.
For more information about the Southeast Asia PSS Skills Exchange held March 22-24, 2023, click here.
To learn more and take action to strengthen pharmaceutical systems in your context, please use the resources below.
Introduction
Why is strengthening pharmaceutical systems critical? Click here to hear MTaPS Director Kofi Aboagye-Nyame speak about the importance of PSS, MTaPS’ approaches to PSS and capacity building, and the key roles that local organizations play.
Part 1
Strengthening Regulatory Systems
Presentation Video
Technical Brief
Presentation
Safety Monitoring of Medicinal Products: Strengthening Pharmacovigilance Systems
Presentation Video
Technical Brief
Presentation
Strengthening Regulatory Information Management Systems
Presentation Video
Technical Brief
Presentation
Part 2
Ensuring Quality and Safety in Pharmaceutical Systems: The Role of Good Governance
Presentation Video
Technical Brief
Presentation
Pharmaceutical Procurement and Supply Chain Management
Presentation Video
Technical Brief
Presentation
Part 3
Pharmaceutical System Financing
Presentation Video
Technical Brief
Presentation
Using Health Technology Assessment to Strengthen Informed Decision Making
Presentation Video
Technical Brief
Presentation
Containing Antimicrobial Resistance Through Stewardship
Presentation Video
Technical Brief
Presentation
Further Local Application Through Mentoring
Building on the regional PSS skills exchanges in East Africa and Southeast Asia, MTaPS offered a follow-on mentoring program to allow participants to delve further into topics of interest with coaches from MTaPS. On July 26, 2023, MTaPS held a report out session to allow the participants and coaches to reflect on their experiences with the mentoring program. Selected participants—representing diverse organizations working in Kenya, Nigeria, Tanzania, Uganda, and Zimbabwe—gave presentations on how they have applied the coaching to their work, demonstrating significant workplace actions linked to the mentoring experience. The program showed the value of using a PSS approach to sustainably address pharmaceutical management problems and strengthen health systems.
To view the recording of the report out session, please click here.
